In some ways, Chemistry is the science of the invisible. There’s simply no way to see things like atoms and molecules with the naked eye—they’re just too small. So how do people know what a molecule really looks like? And how can scientists prove that a molecule is what they say it is?
Structural chemistry is the science of finding out what things look like on a molecular level, and it is sort of like being a detective. Because scientists can’t observe atoms and molecules directly, chemists have to use indirect methods to gather evidence and make a case for their structure. Some chemists spend their careers doing this. They might work to isolate compounds from herbs that have been found to have healing properties so that they can be replicated in pharmaceuticals, or they might do things like test nutritional supplements to see if they really contain the compounds stated.
There are a lot of ways to test a compound to learn about its structure, components, and properties. You can observe its more visible properties, like color, density, and how it reacts with other chemicals. You can test its melting point, boiling point, and what it looks like when it crystalizes. But the most effective weapon in the chemical detective’s arsenal are spectroscopy and spectrometry.
Overview
There are two main kinds of spectroscopy used in organic chemistry: Infrared and Nuclear Magnetic Resonance. These help chemists isolate the bond structure of a molecule and identify the individual components. Mass Spectrometry isolates the molecular mass of a compound.
There are other investigative tools available, like X-Ray crystallography, that are used in other kinds of chemistry to give more details about complicated molecular structure, but these three are sufficient for many purposes.
The Basics of Light
Spectroscopy relies on beams of light, which is a form of radiation. Radiation is made up of photons, which behave both as particles and as waves of energy. Each wavelength of light corresponds to a different energy.
Remember the colors of the rainbow? Low-energy radiation falls on the red end of the spectrum, including things like infrared and microwaves, which are invisible to the naked eye and lie to the left of the visible spectrum. High-energy forms of light fall to the right and include short wavelengths like blue and ultra-violet light.
This can be a bit confusing, because short wavelengths correspond to high energy and long wavelengths correspond to low. One way to remember which is which is that UV light is dangerous for your skin—that’s why staying out in the sun too long is a bad idea. This high-energy, dangerous light pounds your skin with extremely short wavelengths, breaking down and damaging the cells.
Infrared, on the other hand, is used for things like remote controls and computer mice. It’s much less dangerous. These long, lazy wavelengths just don’t have enough energy to do much damage.
Infrared
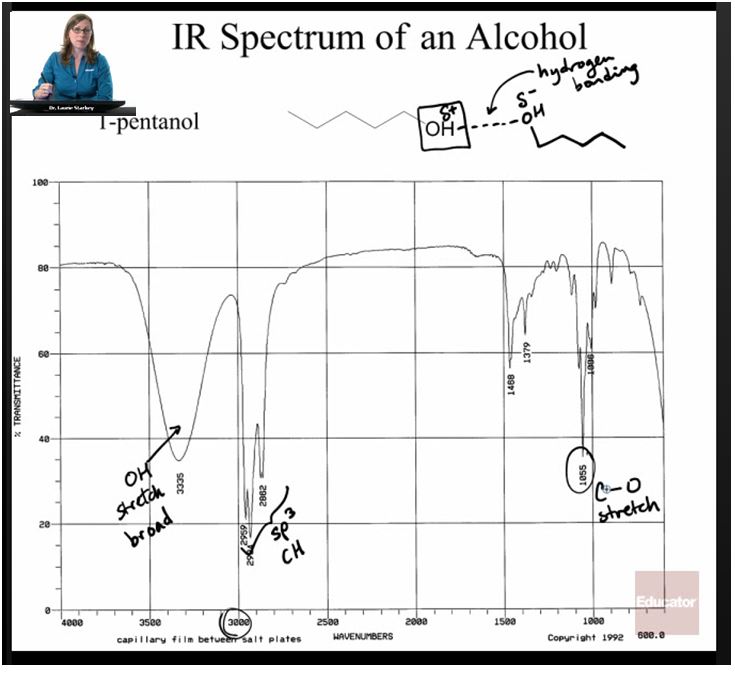
IR spectroscopy uses infrared radiation to excite the bonds in a molecule. IR doesn’t have enough energy to break the bonds, but it can “excite” them when it’s absorbed by the molecule, causing them to vibrate, stretching, bending, or shrinking like a spring.
But different types of bonds require different amounts of energy to vibrate. This means that only certain wavelengths of infrared light are absorbed. By figuring out which wavelengths are absorbed, we can tell which functional groups a molecule has. This works well for groups like OH, alkanes, alkenes, and amines, because they all have polar bonds.
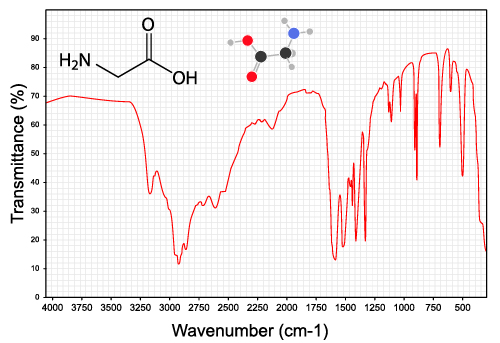
An IR spectrum plots the transmittance—how much light makes it through the sample—against the wavenumber, which is basically the inverse of wavelength. The mostly straight line at the top indicates that light at that wavelength is passing through the sample, while a dip means there was an absorption.
In this plot, there is a broad absorption around 3350 cm-1 showing where the O-H bond absorbed the radiation and began to stretch. There are also several sharper peaks around 3000. These are C-H bond stretches, which indicates the presence of alkane groups in the rest of the molecule. There are also some “fingerprint” peaks at low energy that can be hard to isolate. Computers are often used to identify this region.
IR has one big limitation: it only works with vibrations that change the dipole of the molecule, so non-polar molecules and vibrations that don’t change the dipole won’t result in an IR signal.
Nuclear Magnetic Resonance
To perform NMR spectroscopy, a sample is prepared in a tube and dropped into a machine with a very powerful, superconducting magnet. Then, the sample is irradiated with very low-energy radio waves.
Many nuclei (including, most importantly, hydrogen) have a “magnetic moment” – a kind of magnetic dipole. In a normal material, these nuclei are oriented randomly in many different directions. But when they are exposed to the magnetic field, they align themselves either with the field or against it (aligning with it is a lower energy state, so slightly more nuclei will orient themselves with the field). The radiofrequency pulse then flips the nuclei, and when they decay to their original state, they give off a signal that can be measured.
Where exactly this signal shows up on the scale depends on what kind of functional group the hydrogen is attached to. The shift indicates which functional group is present, while the area of the peak (with some modification) describes how many hydrogens are present in that group.

For more details and sample problems, check out this lecture with Dr. Laurie Starkey.
Mass Spectrometry
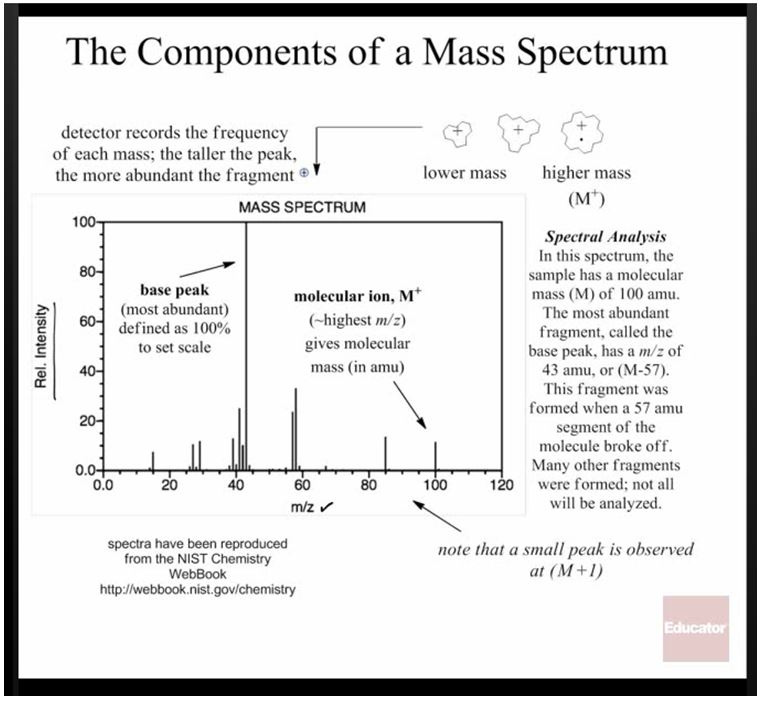
Mass Spec is a little bit different than the other forms of investigation because it doesn’t use light to gain information about a molecule. Instead, it bombards the molecule with electrons, which causes it to break apart into charged radical fragments. These fragments can be separated by a magnet according to their “mass to charge ratio.”
These images are from this lecture on mass spec
This makes it possible to figure out the mass of each of the components, and sometimes even the whole molecule. By calculating the mass of the molecule you expected (adding up the masses of the expected atoms on the periodic table), you can see if the product matches your expectations.
This technique is often combined with gas chromatography and called GC-MS.
Putting It All Together
Once all of these tests have been run, it’s time to play detective and put all the information together. The IR data will show any functional groups or atoms that have a dipolar bond; the NMR spectrum will verify many of these groups if they contain hydrogen; and the mass spec will confirm the total mass of the molecule.
From this information, along with starting materials and some knowledge of organic reaction mechanisms, it’s possible to verify a structure with a pretty good degree of certainty. None of these methods are perfect on their own, but together, they can create a window into the world of things too small to see.
[box type=”success” align=”” class=”” width=””]Check out our lecture set on Organic Chemistry for more examples on Nuclear Magnetic Resonance (NMR) and other spectroscopy subjects.[/box]